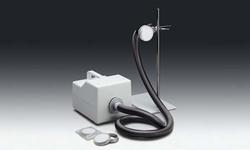
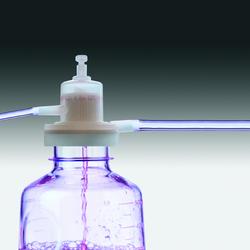
Product Description
Next generation integrity test system
As the requirements on product quality in the pharmaceutical and biotechnology industries increase, so does the need to produce compliant documentation with 100% traceability. This means electronic recording, processing and archiving of data are becoming a necessity.
Sartocheck 4 enables you to keep a step ahead of this trend. This leading edge filter integrity tester gives you full electronic capabilities for all integrity test methods.
Sartocheck 4 allows you to save all program parameters and test results. In addition, our SartoControl data transfer and processing software simplifies back up of all Sartocheck 4 data. Every user action is recorded in an audit trail – in full compliance with 21 CFR Part 11.
Sartocheck 4 has been strictly designed according to current GAMP guidelines. With all of these attributes, you will understand why we’re convinced that we are offering you a “touch of real class” – quality that you can put to the test in your process chain.
Touch Screen
Trouble free cleaning and drying
Data security – up to 350 test programs and 500 test results
Training and assistance with validation documents available